Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(Continued)<br />
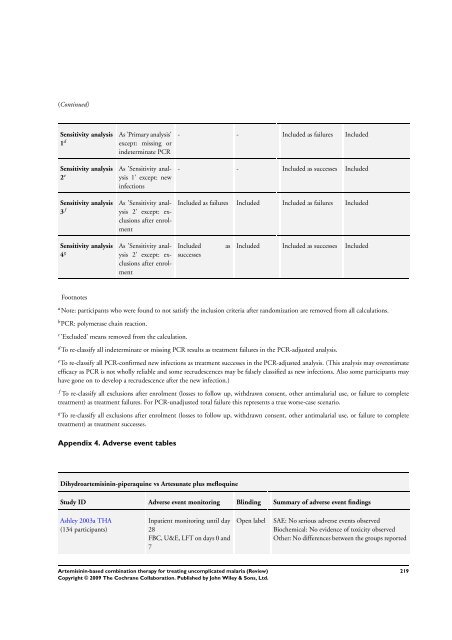
Sensitivity analysis<br />
1 d<br />
Sensitivity analysis<br />
2 e<br />
Sensitivity analysis<br />
3 f<br />
Sensitivity analysis<br />
4 g<br />
Footnotes<br />
As ’Primary analysis’<br />
except: missing or<br />
indeterminate PCR<br />
As ’Sensitivity analysis<br />
1’ except: new<br />
infections<br />
As ’Sensitivity analysis<br />
2’ except: exclusions<br />
after enrolment<br />
As ’Sensitivity analysis<br />
2’ except: exclusions<br />
after enrolment<br />
- - Included as failures Included<br />
- - Included as successes Included<br />
Included as failures Included Included as failures Included<br />
Included as<br />
successes<br />
Included Included as successes Included<br />
a Note: participants who were found to not satisfy the inclusion criteria after randomization are removed from all calculations.<br />
b PCR: polymerase chain reaction.<br />
c ’Excluded’ means removed from the calculation.<br />
d To re-classify all indeterminate or missing PCR results as treatment failures in the PCR-adjusted analysis.<br />
e To re-classify all PCR-confirmed new infections as treatment successes in the PCR-adjusted analysis. (This analysis may overestimate<br />
efficacy as PCR is not wholly reliable and some recrudescences may be falsely classified as new infections. Also some participants may<br />
have gone on to develop a recrudescence after the new infection.)<br />
f To re-classify all exclusions after enrolment (losses to follow up, withdrawn consent, other antimalarial use, or failure to complete<br />
treatment) as treatment failures. For PCR-unadjusted total failure this represents a true worse-case scenario.<br />
g To re-classify all exclusions after enrolment (losses to follow up, withdrawn consent, other antimalarial use, or failure to complete<br />
treatment) as treatment successes.<br />
Appendix 4. Adverse event tables<br />
Dihydroartemisinin-piperaquine vs Artesunate plus mefloquine<br />
Study ID Adverse event monitoring Blinding Summary of adverse event findings<br />
Ashley 2003a THA<br />
(134 participants)<br />
Inpatient monitoring until day<br />
28<br />
FBC, U&E, LFT on days 0 and<br />
7<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
Open label SAE: No serious adverse events observed<br />
Biochemical: No evidence of toxicity observed<br />
Other: No differences between the groups reported<br />
219