Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(Continued)<br />
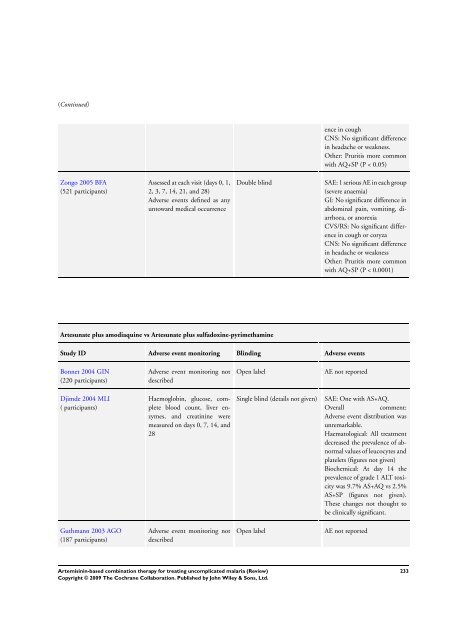
Zongo 2005 BFA<br />
(521 participants)<br />
Assessed at each visit (days 0, 1,<br />
2, 3, 7, 14, 21, and 28)<br />
Adverse events defined as any<br />
untoward medical occurrence<br />
Artesunate plus amodiaquine vs Artesunate plus sulfadoxine-pyrimethamine<br />
ence in cough<br />
CNS: No significant difference<br />
in headache or weakness.<br />
Other: Pruritis more common<br />
with AQ+SP (P < 0.05)<br />
Double blind SAE: 1 serious AE in each group<br />
(severe anaemia)<br />
GI: No significant difference in<br />
abdominal pain, vomiting, diarrhoea,<br />
or anorexia<br />
CVS/RS: No significant difference<br />
in cough or coryza<br />
CNS: No significant difference<br />
in headache or weakness<br />
Other: Pruritis more common<br />
with AQ+SP (P < 0.0001)<br />
Study ID Adverse event monitoring Blinding Adverse events<br />
Bonnet 2004 GIN<br />
(220 participants)<br />
Djimde 2004 MLI<br />
( participants)<br />
Guthmann 2003 AGO<br />
(187 participants)<br />
Adverse event monitoring not<br />
described<br />
Haemoglobin, glucose, complete<br />
blood count, liver enzymes,<br />
and creatinine were<br />
measured on days 0, 7, 14, and<br />
28<br />
Adverse event monitoring not<br />
described<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
Open label AE not reported<br />
Single blind (details not given) SAE: One with AS+AQ.<br />
Overall comment:<br />
Adverse event distribution was<br />
unremarkable.<br />
Haematological: All treatment<br />
decreased the prevalence of abnormal<br />
values of leucocytes and<br />
platelets (figures not given)<br />
Biochemical: At day 14 the<br />
prevalence of grade 1 ALT toxicity<br />
was 9.7% AS+AQ vs 2.5%<br />
AS+SP (figures not given).<br />
<strong>The</strong>se changes not thought to<br />
be clinically significant.<br />
Open label AE not reported<br />
233