Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
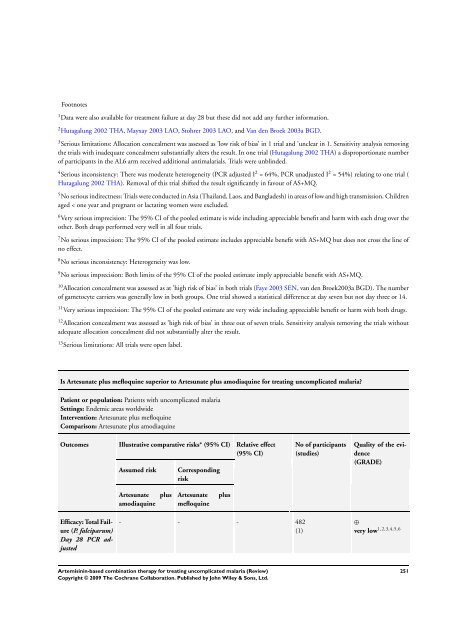
Footnotes<br />
1 Data were also available <strong>for</strong> treatment failure at day 28 but these did not add any further in<strong>for</strong>mation.<br />
2 Hutagalung 2002 THA, Mayxay 2003 LAO, Stohrer 2003 LAO, and Van den Broek 2003a BGD.<br />
3 Serious limitations: Allocation concealment was assessed as ’low risk of bias’ in 1 trial and ’unclear in 1. Sensitivity analysis removing<br />
the trials with inadequate concealment substantially alters the result. In one trial (Hutagalung 2002 THA) a disproportionate number<br />
of participants in the AL6 arm received additional antimalarials. Trials were unblinded.<br />
4 Serious inconsistency: <strong>The</strong>re was moderate heterogeneity (PCR adjusted I 2 = 64%, PCR unadjusted I 2 = 54%) relating to one trial (<br />
Hutagalung 2002 THA). Removal of this trial shifted the result significantly in favour of AS+MQ.<br />
5 No serious indirectness: Trials were conducted in Asia (Thailand, Laos, and Bangladesh) in areas of low and high transmission. Children<br />
aged < one year and pregnant or lactating women were excluded.<br />
6 Very serious imprecision: <strong>The</strong> 95% CI of the pooled estimate is wide including appreciable benefit and harm with each drug over the<br />
other. Both drugs per<strong>for</strong>med very well in all four trials.<br />
7 No serious imprecision: <strong>The</strong> 95% CI of the pooled estimate includes appreciable benefit with AS+MQ but does not cross the line of<br />
no effect.<br />
8 No serious inconsistency: Heterogeneity was low.<br />
9 No serious imprecision: Both limits of the 95% CI of the pooled estimate imply appreciable benefit with AS+MQ.<br />
10 Allocation concealment was assessed as at ’high risk of bias’ in both trials (Faye 2003 SEN, van den Broek2003a BGD). <strong>The</strong> number<br />
of gametocyte carriers was generally low in both groups. One trial showed a statistical difference at day seven but not day three or 14.<br />
11 Very serious imprecision: <strong>The</strong> 95% CI of the pooled estimate are very wide including appreciable benefit or harm with both drugs.<br />
12 Allocation concealment was assessed as ’high risk of bias’ in three out of seven trials. Sensitivity analysis removing the trials without<br />
adequate allocation concealment did not substantially alter the result.<br />
13 Serious limitations: All trials were open label.<br />
Is Artesunate plus mefloquine superior to Artesunate plus amodiaquine <strong>for</strong> treating uncomplicated malaria?<br />
Patient or population: Patients with uncomplicated malaria<br />
Settings: Endemic areas worldwide<br />
Intervention: Artesunate plus mefloquine<br />
Comparison: Artesunate plus amodiaquine<br />
Outcomes Illustrative comparative risks* (95% CI) Relative effect<br />
(95% CI)<br />
Efficacy: Total Failure<br />
(P. falciparum)<br />
Day 28 PCR adjusted<br />
Assumed risk Corresponding<br />
risk<br />
Artesunate plus<br />
amodiaquine<br />
Artesunate plus<br />
mefloquine<br />
- - - 482<br />
(1)<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
No of participants<br />
(studies)<br />
Quality of the evidence<br />
(GRADE)<br />
⊕<br />
very low 1,2,3,4,5,6<br />
251