Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
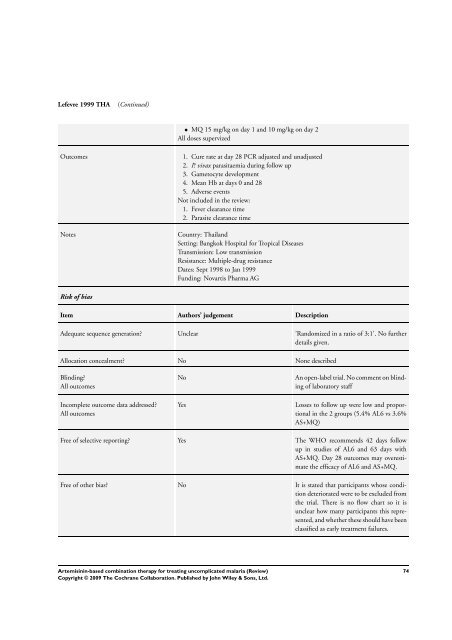
Lefevre 1999 THA (Continued)<br />
• MQ 15 mg/kg on day 1 and 10 mg/kg on day 2<br />
All doses supervized<br />
Outcomes 1. Cure rate at day 28 PCR adjusted and unadjusted<br />
2. P. vivax parasitaemia during follow up<br />
3. Gametocyte development<br />
4. Mean Hb at days 0 and 28<br />
5. Adverse events<br />
Not included in the review:<br />
1. Fever clearance time<br />
2. Parasite clearance time<br />
Notes Country: Thailand<br />
Setting: Bangkok Hospital <strong>for</strong> Tropical Diseases<br />
Transmission: Low transmission<br />
Resistance: Multiple-drug resistance<br />
Dates: Sept 1998 to Jan 1999<br />
Funding: Novartis Pharma AG<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Unclear ’Randomized in a ratio of 3:1’. No further<br />
details given.<br />
Allocation concealment? No None described<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No An open-label trial. No comment on blinding<br />
of laboratory staff<br />
Yes Losses to follow up were low and proportional<br />
in the 2 groups (5.4% AL6 vs 3.6%<br />
AS+MQ)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow<br />
up in studies of AL6 and 63 days with<br />
AS+MQ. Day 28 outcomes may overestimate<br />
the efficacy of AL6 and AS+MQ.<br />
Free of other bias? No It is stated that participants whose condition<br />
deteriorated were to be excluded from<br />
the trial. <strong>The</strong>re is no flow chart so it is<br />
unclear how many participants this represented,<br />
and whether these should have been<br />
classified as early treatment failures.<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
74