Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
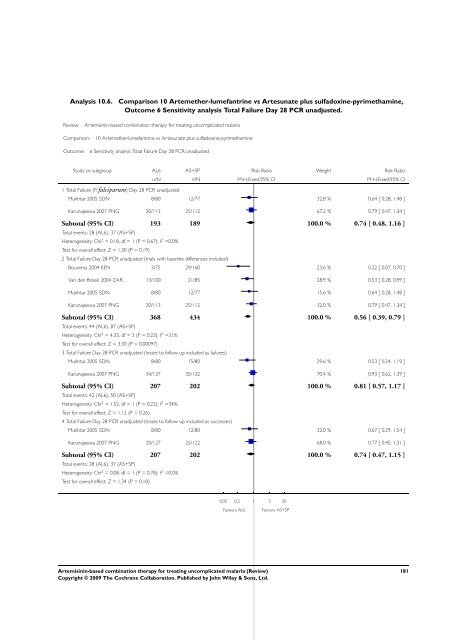
Analysis 10.6. Comparison 10 Artemether-lumefantrine vs Artesunate plus sulfadoxine-pyrimethamine,<br />
Outcome 6 Sensitivity analysis Total Failure Day 28 PCR unadjusted.<br />
Review: <strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria<br />
Comparison: 10 Artemether-lumefantrine vs Artesunate plus sulfadoxine-pyrimethamine<br />
Outcome: 6 Sensitivity analysis Total Failure Day 28 PCR unadjusted<br />
Study or subgroup AL6 AS+SP Risk Ratio Weight Risk Ratio<br />
1 Total Failure (P. falciparum) Day 28 PCR unadjusted<br />
n/N n/N M-H,Fixed,95% CI M-H,Fixed,95% CI<br />
Mukhtar 2005 SDN 8/80 12/77 32.8 % 0.64 [ 0.28, 1.48 ]<br />
Karunajeewa 2007 PNG 20/113 25/112 67.2 % 0.79 [ 0.47, 1.34 ]<br />
Subtotal (95% CI) 193 189 100.0 % 0.74 [ 0.48, 1.16 ]<br />
Total events: 28 (AL6), 37 (AS+SP)<br />
Heterogeneity: Chi 2 = 0.18, df = 1 (P = 0.67); I 2 =0.0%<br />
Test <strong>for</strong> overall effect: Z = 1.30 (P = 0.19)<br />
2 Total Failure Day 28 PCR unadjusted (trials with baseline differences included)<br />
Bousema 2004 KEN 3/75 29/160 23.6 % 0.22 [ 0.07, 0.70 ]<br />
Van den Broek 2004 ZAR 13/100 21/85 28.9 % 0.53 [ 0.28, 0.99 ]<br />
Mukhtar 2005 SDN 8/80 12/77 15.6 % 0.64 [ 0.28, 1.48 ]<br />
Karunajeewa 2007 PNG 20/113 25/112 32.0 % 0.79 [ 0.47, 1.34 ]<br />
Subtotal (95% CI) 368 434 100.0 % 0.56 [ 0.39, 0.79 ]<br />
Total events: 44 (AL6), 87 (AS+SP)<br />
Heterogeneity: Chi 2 = 4.33, df = 3 (P = 0.23); I 2 =31%<br />
Test <strong>for</strong> overall effect: Z = 3.30 (P = 0.00097)<br />
3 Total Failure Day 28 PCR unadjusted (losses to follow up included as failures)<br />
Mukhtar 2005 SDN 8/80 15/80 29.6 % 0.53 [ 0.24, 1.19 ]<br />
Karunajeewa 2007 PNG 34/127 35/122 70.4 % 0.93 [ 0.62, 1.39 ]<br />
Subtotal (95% CI) 207 202 100.0 % 0.81 [ 0.57, 1.17 ]<br />
Total events: 42 (AL6), 50 (AS+SP)<br />
Heterogeneity: Chi 2 = 1.52, df = 1 (P = 0.22); I 2 =34%<br />
Test <strong>for</strong> overall effect: Z = 1.12 (P = 0.26)<br />
4 Total Failure Day 28 PCR unadjusted (losses to follow up included as successes)<br />
Mukhtar 2005 SDN 8/80 12/80 32.0 % 0.67 [ 0.29, 1.54 ]<br />
Karunajeewa 2007 PNG 20/127 25/122 68.0 % 0.77 [ 0.45, 1.31 ]<br />
Subtotal (95% CI) 207 202 100.0 % 0.74 [ 0.47, 1.15 ]<br />
Total events: 28 (AL6), 37 (AS+SP)<br />
Heterogeneity: Chi 2 = 0.08, df = 1 (P = 0.78); I 2 =0.0%<br />
Test <strong>for</strong> overall effect: Z = 1.34 (P = 0.18)<br />
0.05 0.2 1 5 20<br />
Favours AL6 Favours AS+SP<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
181