Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
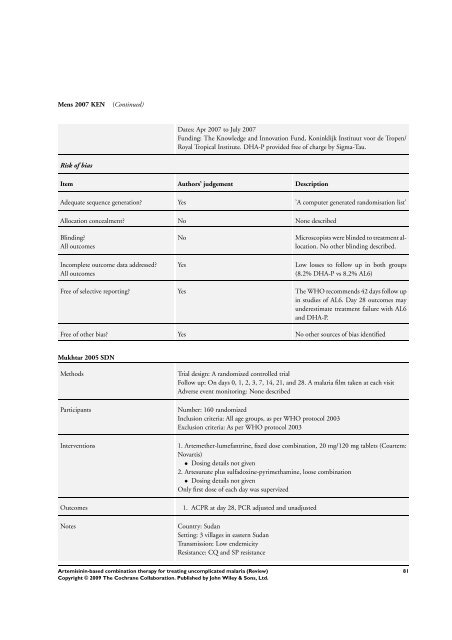
Mens 2007 KEN (Continued)<br />
Risk of bias<br />
Dates: Apr 2007 to July 2007<br />
Funding: <strong>The</strong> Knowledge and Innovation Fund, Koninklijk Instituut voor de Tropen/<br />
Royal Tropical Institute. DHA-P provided free of charge by Sigma-Tau.<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’A computer generated randomisation list’<br />
Allocation concealment? No None described<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
No Microscopists were blinded to treatment allocation.<br />
No other blinding described.<br />
Yes Low losses to follow up in both groups<br />
(8.2% DHA-P vs 8.2% AL6)<br />
Free of selective reporting? Yes <strong>The</strong> WHO recommends 42 days follow up<br />
in studies of AL6. Day 28 outcomes may<br />
underestimate treatment failure with AL6<br />
and DHA-P.<br />
Free of other bias? Yes No other sources of bias identified<br />
Mukhtar 2005 SDN<br />
Methods Trial design: A randomized controlled trial<br />
Follow up: On days 0, 1, 2, 3, 7, 14, 21, and 28. A malaria film taken at each visit<br />
Adverse event monitoring: None described<br />
Participants Number: 160 randomized<br />
Inclusion criteria: All age groups, as per WHO protocol 2003<br />
Exclusion criteria: As per WHO protocol 2003<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• Dosing details not given<br />
2. Artesunate plus sulfadoxine-pyrimethamine, loose <strong>combination</strong><br />
• Dosing details not given<br />
Only first dose of each day was supervized<br />
Outcomes 1. ACPR at day 28, PCR adjusted and unadjusted<br />
Notes Country: Sudan<br />
Setting: 3 villages in eastern Sudan<br />
Transmission: Low endemicity<br />
Resistance: CQ and SP resistance<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
81