Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
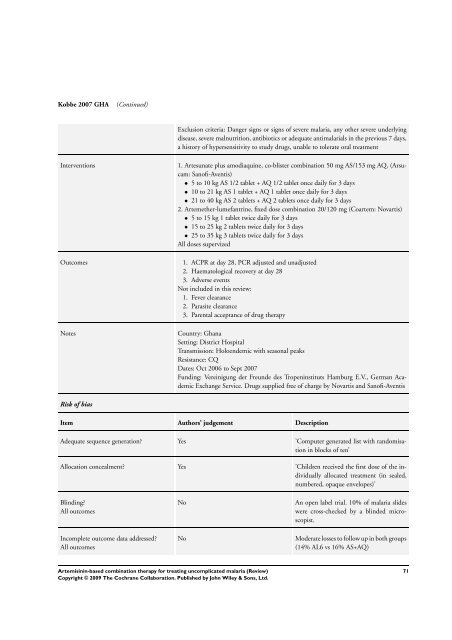
Kobbe 2007 GHA (Continued)<br />
Exclusion criteria: Danger signs or signs of severe malaria, any other severe underlying<br />
disease, severe malnutrition, antibiotics or adequate antimalarials in the previous 7 days,<br />
a history of hypersensitivity to study drugs, unable to tolerate oral treatment<br />
Interventions 1. Artesunate plus amodiaquine, co-blister <strong>combination</strong> 50 mg AS/153 mg AQ, (Arsucam:<br />
Sanofi-Aventis)<br />
• 5 to 10 kg AS 1/2 tablet + AQ 1/2 tablet once daily <strong>for</strong> 3 days<br />
• 10 to 21 kg AS 1 tablet + AQ 1 tablet once daily <strong>for</strong> 3 days<br />
• 21 to 40 kg AS 2 tablets + AQ 2 tablets once daily <strong>for</strong> 3 days<br />
2. Artemether-lumefantrine, fixed dose <strong>combination</strong> 20/120 mg (Coartem: Novartis)<br />
• 5 to 15 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 25 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 35 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
All doses supervized<br />
Outcomes 1. ACPR at day 28, PCR adjusted and unadjusted<br />
2. Haematological recovery at day 28<br />
3. Adverse events<br />
Not included in this review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
3. Parental acceptance of drug <strong>therapy</strong><br />
Notes Country: Ghana<br />
Setting: District Hospital<br />
Transmission: Holoendemic with seasonal peaks<br />
Resistance: CQ<br />
Dates: Oct 2006 to Sept 2007<br />
Funding: Vereinigung der Freunde des Tropeninstituts Hamburg E.V., German Academic<br />
Exchange Service. Drugs supplied free of charge by Novartis and Sanofi-Aventis<br />
Risk of bias<br />
Item Authors’ judgement Description<br />
Adequate sequence generation? Yes ’Computer generated list with randomisation<br />
in blocks of ten’<br />
Allocation concealment? Yes ’Children received the first dose of the individually<br />
allocated treatment (in sealed,<br />
numbered, opaque envelopes)’<br />
Blinding?<br />
All outcomes<br />
Incomplete outcome data addressed?<br />
All outcomes<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
No An open label trial. 10% of malaria slides<br />
were cross-checked by a blinded microscopist.<br />
No Moderate losses to follow up in both groups<br />
(14% AL6 vs 16% AS+AQ)<br />
71