Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Artemisinin-based combination therapy for ... - The Cochrane Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
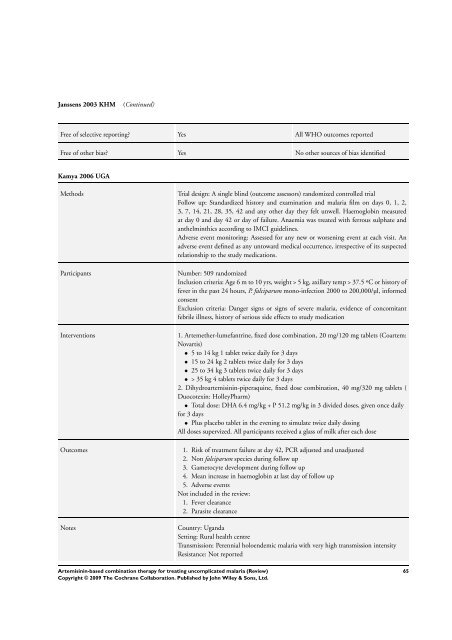
Janssens 2003 KHM (Continued)<br />
Free of selective reporting? Yes All WHO outcomes reported<br />
Free of other bias? Yes No other sources of bias identified<br />
Kamya 2006 UGA<br />
Methods Trial design: A single blind (outcome assessors) randomized controlled trial<br />
Follow up: Standardized history and examination and malaria film on days 0, 1, 2,<br />
3, 7, 14, 21, 28, 35, 42 and any other day they felt unwell. Haemoglobin measured<br />
at day 0 and day 42 or day of failure. Anaemia was treated with ferrous sulphate and<br />
anthelminthics according to IMCI guidelines.<br />
Adverse event monitoring: Assessed <strong>for</strong> any new or worsening event at each visit. An<br />
adverse event defined as any untoward medical occurrence, irrespective of its suspected<br />
relationship to the study medications.<br />
Participants Number: 509 randomized<br />
Inclusion criteria: Age 6 m to 10 yrs, weight > 5 kg, axillary temp > 37.5 ºC or history of<br />
fever in the past 24 hours, P. falciparum mono-infection 2000 to 200,000/µl, in<strong>for</strong>med<br />
consent<br />
Exclusion criteria: Danger signs or signs of severe malaria, evidence of concomitant<br />
febrile illness, history of serious side effects to study medication<br />
Interventions 1. Artemether-lumefantrine, fixed dose <strong>combination</strong>, 20 mg/120 mg tablets (Coartem:<br />
Novartis)<br />
• 5 to 14 kg 1 tablet twice daily <strong>for</strong> 3 days<br />
• 15 to 24 kg 2 tablets twice daily <strong>for</strong> 3 days<br />
• 25 to 34 kg 3 tablets twice daily <strong>for</strong> 3 days<br />
• > 35 kg 4 tablets twice daily <strong>for</strong> 3 days<br />
2. Dihydroartemisinin-piperaquine, fixed dose <strong>combination</strong>, 40 mg/320 mg tablets (<br />
Duocotexin: HolleyPharm)<br />
• Total dose: DHA 6.4 mg/kg + P 51.2 mg/kg in 3 divided doses, given once daily<br />
<strong>for</strong> 3 days<br />
• Plus placebo tablet in the evening to simulate twice daily dosing<br />
All doses supervized. All participants received a glass of milk after each dose<br />
Outcomes 1. Risk of treatment failure at day 42, PCR adjusted and unadjusted<br />
2. Non falciparum species during follow up<br />
3. Gametocyte development during follow up<br />
4. Mean increase in haemoglobin at last day of follow up<br />
5. Adverse events<br />
Not included in the review:<br />
1. Fever clearance<br />
2. Parasite clearance<br />
Notes Country: Uganda<br />
Setting: Rural health centre<br />
Transmission: Perennial holoendemic malaria with very high transmission intensity<br />
Resistance: Not reported<br />
<strong>Artemisinin</strong>-<strong>based</strong> <strong>combination</strong> <strong>therapy</strong> <strong>for</strong> treating uncomplicated malaria (Review)<br />
Copyright © 2009 <strong>The</strong> <strong>Cochrane</strong> Collaboration. Published by John Wiley & Sons, Ltd.<br />
65