- Page 1 and 2:
Université de Bourgogne Institut U
- Page 3 and 4:
Sommaire Sommaire INTRODUCTION ....
- Page 5 and 6:
Sommaire C. Le sulfitage et les enz
- Page 7 and 8:
Sommaire RESULTATS ................
- Page 9 and 10:
Table des figures mobilisable (ou c
- Page 11 and 12:
Tables des tableaux Table des table
- Page 13 and 14:
Introduction Pour faire face à ce
- Page 15 and 16:
Synthèse bibliographique utilisée
- Page 17 and 18:
Synthèse bibliographique biochimiq
- Page 19 and 20:
Synthèse bibliographique primaires
- Page 21 and 22:
Synthèse bibliographique sont des
- Page 23 and 24:
Synthèse bibliographique V. Foncti
- Page 25 and 26:
Synthèse bibliographique contre un
- Page 27 and 28:
Synthèse bibliographique Amines bi
- Page 29 and 30:
Synthèse bibliographique précurse
- Page 31 and 32:
Synthèse bibliographique mg.kg 1 e
- Page 33 and 34:
Synthèse bibliographique phosphate
- Page 35 and 36:
1) Quantification des amines biogè
- Page 37 and 38:
Synthèse bibliographique appartena
- Page 39 and 40:
E. Le pH Synthèse bibliographique
- Page 41 and 42:
Synthèse bibliographique biogènes
- Page 43 and 44:
Synthèse bibliographique La séque
- Page 45 and 46:
Synthèse bibliographique plus, le
- Page 47 and 48:
Synthèse bibliographique Figure 9.
- Page 49 and 50:
a b Synthèse bibliographique porta
- Page 51 and 52:
Synthèse bibliographique Figure 11
- Page 53 and 54:
Synthèse bibliographique biogènes
- Page 55 and 56:
Chapitre II : Les outils moléculai
- Page 57 and 58:
Synthèse bibliographique M17) et l
- Page 59 and 60:
Synthèse bibliographique Les trans
- Page 61 and 62:
Synthèse bibliographique Figure 14
- Page 63 and 64:
Synthèse bibliographique fonction
- Page 65 and 66: Synthèse bibliographique l’absen
- Page 67 and 68: II. Criblage des souches productric
- Page 69 and 70: Matériels et méthodes 10 µL de r
- Page 71 and 72: Matériels et méthodes (BioRad),
- Page 73 and 74: Matériels et méthodes A. Construc
- Page 75 and 76: Matériels et méthodes réaction d
- Page 77 and 78: Matériels et méthodes autour de l
- Page 79 and 80: Matériels et méthodes un substrat
- Page 81 and 82: Figure 21. Evolution du pourcentage
- Page 83 and 84: Histamine Tryptamine Cadavérine Ma
- Page 85 and 86: Matériels et méthodes sur la colo
- Page 87 and 88: Chapitre III : transcriptomique La
- Page 89 and 90: ΔCT = CT (témoin interne) - CT (g
- Page 91 and 92: RESULTATS Résultats Ma thèse s’
- Page 93 and 94: A. L’histamine dans la résistanc
- Page 95 and 96: Résultats été répétée en mili
- Page 97 and 98: Résultats présence de 25 mM d’h
- Page 99 and 100: Résultats Figure 29. Mesure de l
- Page 101 and 102: Figure 30. Carte du vecteur suicide
- Page 103 and 104: Résultats Suite aux différents é
- Page 105 and 106: Molecular cloning, heterologous exp
- Page 107 and 108: Résultats All fermented foods (dai
- Page 109 and 110: Résultats Madison WI USA), 1.5 µl
- Page 111 and 112: Résultats reaction was started by
- Page 113 and 114: Résultats Figure 1: Geographical d
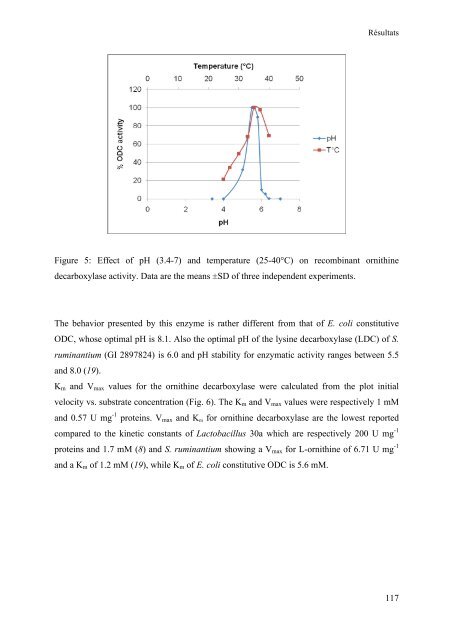
- Page 115: 5 Lactobacillus DFGVPATIVANYLRDHGII
- Page 119 and 120: Résultats enzymes are carried on a
- Page 121 and 122: Résultats 13-Marcobal, A., B. de l
- Page 123 and 124: Article 2 Tyrosine-containing pepti
- Page 125 and 126: Abstract Résultats Biogenic amines
- Page 127 and 128: Résultats Although free AA are pre
- Page 129 and 130: Résultats splitter (split ratio =
- Page 131 and 132: Table 1: Oligonucleotides used in t
- Page 133 and 134: Table 2: Identification of amines b
- Page 135 and 136: Résultats strains, do not carry th
- Page 137 and 138: Résultats (Strahinic et al, 2009),
- Page 139 and 140: Résultats Duary RK, Batish VK & Gr
- Page 141 and 142: Résultats Nannelli F, Claisse O, G
- Page 143 and 144: Discussion-Perspectives décarboxyl
- Page 145 and 146: Discussion-Perspectives plupart du
- Page 147 and 148: ANNEXES Annexes 147
- Page 149 and 150: Annexes d’utiliser cet hôte pour
- Page 151 and 152: Annexes surface des vésicules pert
- Page 153 and 154: Annexes mécanismes, en particulier
- Page 155 and 156: Annexes Afin de vérifier l’effic
- Page 157 and 158: Annexes Marty-Teysset C., Lolkema J
- Page 159 and 160: evis GAAGTTAATCAAGAATTAGTTGCCGGCAAG
- Page 161 and 162: curvatus AGCTGGTAAAGTAACGGTTCTTCGGG
- Page 163 and 164: Références Arena M.E., Manca de N
- Page 165 and 166: Références Bonetta S., Carraro E.
- Page 167 and 168:
Références Capozzi V., Ladero V.,
- Page 169 and 170:
Références Coton M., Fernandez M.
- Page 171 and 172:
Références Endo, A., Okada, S., R
- Page 173 and 174:
Références Gerbaux V., Villa A.,
- Page 175 and 176:
Références Jobin M.-P., Garmyn D.
- Page 177 and 178:
Références Landete J. M., Pardo I
- Page 179 and 180:
Références Lonvaud-Funel A. & Joy
- Page 181 and 182:
Références Marques A. P., Leitao
- Page 183 and 184:
N Références Nakada Y., Itoh Y.,
- Page 185 and 186:
Références Pramateftaki P.V., Met
- Page 187 and 188:
Références Sato T., Fukui T., Ato
- Page 189 and 190:
Références Teissie J., Rols M.P.,
- Page 191 and 192:
W-X Références Wei M.Q., Rush C.M
- Page 193 and 194:
RESUME Résumé Les amines biogène