- Page 2 and 3:
Conservation of Furniture
- Page 4 and 5:
Conservation of Furniture Shayne Ri
- Page 6 and 7:
Contents Series editors’ preface
- Page 8 and 9:
3.2.5 Coated fabrics and ‘leather
- Page 10 and 11:
The actual move 275 Protection of o
- Page 12 and 13:
Recording and reporting treatment 4
- Page 14 and 15:
11.5.6 Enzymes 548 11.5.7 Blanching
- Page 16 and 17:
15.3.5 Repairs 683 15.3.6 Replaceme
- Page 18 and 19:
16.9.2 General care 771 16.9.3 Clea
- Page 20 and 21:
Series editors’ preface The conse
- Page 22 and 23:
Contributors Part 1 History 1 Furni
- Page 24 and 25:
Brenda Keneghan Senior Conservation
- Page 26 and 27:
Acknowledgements It would take a ve
- Page 28 and 29:
Illustration acknowledgements The a
- Page 30 and 31:
Figure 4.14 (a,c,d) Drawing by Liz
- Page 32 and 33:
Figure 11.9 Julie Arslano˘glu base
- Page 34 and 35:
Figure 15.17 Courtesy of P.R. Jacks
- Page 36 and 37:
Part 1 History
- Page 38 and 39:
1 Furniture history 1.1 Introductio
- Page 40 and 41:
Figure 1.2 Klismos chair, English,
- Page 42 and 43:
Figure 1.3 Late medieval oak Englis
- Page 44 and 45:
conjunction of polychromy and carvi
- Page 46 and 47:
Materials used The choice of materi
- Page 48 and 49:
period reflects the dour and simple
- Page 50 and 51:
Figure 1.10 High-backed cane chair,
- Page 52 and 53:
to use the continental dovetailing
- Page 54 and 55:
Figure 1.14 Japanned cabinet on sta
- Page 56 and 57:
furniture by contrast was generally
- Page 58 and 59:
Figure 1.16 A mahogany English Rege
- Page 60 and 61:
One process of construction that co
- Page 62 and 63:
eforms in education (1870 Elementar
- Page 64 and 65:
chesterfields, chiffoniers, davenpo
- Page 66 and 67:
Figure 1.20 Papier mâché chair, E
- Page 68 and 69:
Figure 1.21 Thonet bentwood chair,
- Page 70 and 71:
major feature, and confirmed the se
- Page 72 and 73:
Figure 1.25 Sideboard, ‘Casablanc
- Page 74 and 75:
Figure 1.27 Tulip chair, designed b
- Page 76 and 77:
The range of finishes has increased
- Page 78 and 79:
Viaux, J. (1962) Le Meuble en Franc
- Page 80 and 81:
Specific texts relating to trade or
- Page 82 and 83:
Part 2 Materials
- Page 84 and 85:
2 Wood and wooden structures It is
- Page 86 and 87:
many properties, such as increasing
- Page 88 and 89:
ant consideration in the selection
- Page 90 and 91:
2.2.2 Wood anatomy: softwoods The c
- Page 92 and 93:
may be gradual in some woods, abrup
- Page 94 and 95:
PARENCHYMA ARRANGEMENTS V P Apotrac
- Page 96 and 97:
the United States. In the UK, the T
- Page 98 and 99:
Ash - Fraxinus spp. (1) F. american
- Page 100 and 101:
Birch - Betula spp. (1) B. alleghan
- Page 102 and 103:
Rosewood - Dalbergia spp. D latifol
- Page 104 and 105:
A A northern red oak Quercus rubra
- Page 106 and 107:
Figure 2.9 Tangential microscopic v
- Page 108 and 109:
In the walnut genus, Juglans, two i
- Page 110 and 111:
Figure 2.12 A representative portio
- Page 112 and 113:
depending upon species, whether sap
- Page 114 and 115:
0 and 25% moisture content. They ha
- Page 116 and 117:
Equilibrium moisture content (%) 28
- Page 118 and 119:
mechanical effects of repeated shri
- Page 120 and 121:
wood member, for example, a block o
- Page 122 and 123:
The strength of wood in compression
- Page 124 and 125:
er of defects and repairs, and the
- Page 126 and 127:
unwanted movement in the joint is r
- Page 128 and 129:
2.7.5 Other joint types Among other
- Page 130 and 131:
Figure 2.30 Dovetails and associate
- Page 132 and 133:
3 Upholstery materials and structur
- Page 134 and 135:
Figure 3.1 A late sixteenth century
- Page 136 and 137:
Epidermis/ skin Hair shaft Sweat gl
- Page 138 and 139:
it over a blunt metal blade or by p
- Page 140 and 141:
and dried to remove unwanted flesh
- Page 142 and 143:
Rattan or cane The cane that is use
- Page 144 and 145:
wood pulp and cellulose acetate fro
- Page 146 and 147:
The surfaces of both woven and non-
- Page 148 and 149:
(Gill and Eastop, 2001). ‘Throwaw
- Page 150 and 151:
was not until the early nineteenth
- Page 152 and 153:
(a) (b) (c) Horse hair, obtained fr
- Page 154 and 155:
The ability of elastomers to be mou
- Page 156 and 157:
include polysaccharide gums such as
- Page 158 and 159:
Shearer, G.L. (1989) An Evaluation
- Page 160 and 161:
Plastics and polymers, coatings and
- Page 162 and 163:
Plastics and polymers, coatings and
- Page 164 and 165:
Plastics and polymers, coatings and
- Page 166 and 167:
Melamine formaldehyde (MF) Phenol f
- Page 168 and 169:
Polyurethanes (PUR) Poly(vinyl acet
- Page 170 and 171:
Plastics and polymers, coatings and
- Page 172 and 173:
Plastics and polymers, coatings and
- Page 174 and 175:
Plastics and polymers, coatings and
- Page 176 and 177:
Plastics and polymers, coatings and
- Page 178 and 179:
Plastics and polymers, coatings and
- Page 180 and 181:
Plastics and polymers, coatings and
- Page 182 and 183:
Plastics and polymers, coatings and
- Page 184 and 185:
Plastics and polymers, coatings and
- Page 186 and 187:
Plastics and polymers, coatings and
- Page 188 and 189:
Plastics and polymers, coatings and
- Page 190 and 191:
Plastics and polymers, coatings and
- Page 192 and 193:
Plastics and polymers, coatings and
- Page 194 and 195:
Plastics and polymers, coatings and
- Page 196 and 197:
Plastics and polymers, coatings and
- Page 198 and 199:
Plastics and polymers, coatings and
- Page 200 and 201:
CH 2—OOCR l R ll COO—CH O CH
- Page 202 and 203:
Plastics and polymers, coatings and
- Page 204 and 205:
(c) 4.7.4 Proteins Plastics and pol
- Page 206 and 207:
Plastics and polymers, coatings and
- Page 208 and 209:
Plastics and polymers, coatings and
- Page 210 and 211:
Plastics and polymers, coatings and
- Page 212 and 213:
Triterpenoids Plastics and polymers
- Page 214 and 215:
Plastics and polymers, coatings and
- Page 216 and 217: Plastics and polymers, coatings and
- Page 218 and 219: Plastics and polymers, coatings and
- Page 220 and 221: Plastics and polymers, coatings and
- Page 222 and 223: Plastics and polymers, coatings and
- Page 224 and 225: Plastics and polymers, coatings and
- Page 226 and 227: Plastics and polymers, coatings and
- Page 228 and 229: Plastics and polymers, coatings and
- Page 230 and 231: Figure 5.2 Sources of ivory that ha
- Page 232 and 233: (a) (b) Bone and antler Bone has be
- Page 234 and 235: Table 5.1 Class characteristics of
- Page 236 and 237: In walrus ivory an outer primary de
- Page 238 and 239: (a) (b) imitate the more costly tur
- Page 240 and 241: Figure 5.12 Mollusc shells used in
- Page 242 and 243: tive applications. Metal tools have
- Page 244 and 245: e preferred by hand smiths until it
- Page 246 and 247: Table 5.2 Relationship between comp
- Page 248 and 249: observed features can be carried ou
- Page 250 and 251: Figure 5.14 The use of a crane to m
- Page 252 and 253: Early in the fourteenth century sma
- Page 254 and 255: diffraction, energy dispersive X-ra
- Page 256 and 257: a substance exists as a hybrid of t
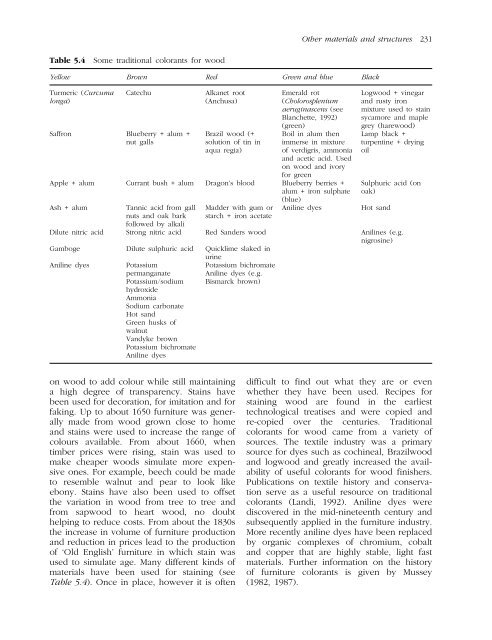
- Page 258 and 259: Table 5.3 Pigments Other materials
- Page 260 and 261: Table 5.3 Pigments - continued Othe
- Page 262 and 263: Table 5.3 Pigments - continued Othe
- Page 264 and 265: Table 5.3 Pigments - continued will
- Page 268 and 269: parent to X-rays. X-ray diffraction
- Page 270 and 271: Larsen, E.B. (1984) Electrotyping,
- Page 272 and 273: Van Duin, P. (1989) Two pairs of Bo
- Page 274 and 275: Part 3 Deterioration
- Page 276 and 277: 6 General review of environment and
- Page 278 and 279: which water, oxygen and other react
- Page 280 and 281: standard free energies of starting
- Page 282 and 283: protect the material. When these be
- Page 284 and 285: Table 6.1 UV Component of various l
- Page 286 and 287: through which daylight is highly di
- Page 288 and 289: climate. An important aspect of the
- Page 290 and 291: (a) 60 (b) 30 55 30 50 45 25 40 35
- Page 292 and 293: panying fungal attack. Animal fibre
- Page 294 and 295: allowed to expand in one part of th
- Page 296 and 297: paint, silk and some dyes and pigme
- Page 298 and 299: ences listed at the end of the foll
- Page 300 and 301: about by localized conditions such
- Page 302 and 303: about the same size as the width of
- Page 304 and 305: ing for some time before they are n
- Page 306 and 307: may cause irreparable damage. Appli
- Page 308 and 309: ensure that the chance of further i
- Page 310 and 311: plied by the density of the materia
- Page 312 and 313: 6.2.8 Environmental management for
- Page 314 and 315: est changed at the same intervals.
- Page 316 and 317:
in locations familiar to all to sav
- Page 318 and 319:
Appelbaum, B. (1992) Guide to Envir
- Page 320 and 321:
7 Deterioration of wood and wooden
- Page 322 and 323:
(a) Figure 7.1 A knot is a portion
- Page 324 and 325:
figure or behaviour. Tangential (fl
- Page 326 and 327:
time of exposure, moisture content
- Page 328 and 329:
RH changes lead to changes in moist
- Page 330 and 331:
Equilibrium Moisture Content (EMC)
- Page 332 and 333:
(a) (b) Figure 7.6 The common furni
- Page 334 and 335:
one has proof of active infestation
- Page 336 and 337:
including starch depletion of timbe
- Page 338 and 339:
ise, when making wooden structures,
- Page 340 and 341:
(a) (b) (a) (b) Faults in execution
- Page 342 and 343:
Figure 7.14 Rear door of a boulle b
- Page 344 and 345:
Relative humidity (%) 100 90 80 70
- Page 346 and 347:
woodworm damage is accompanied by s
- Page 348 and 349:
century. In between these examples
- Page 350 and 351:
8 Deterioration of other materials
- Page 352 and 353:
(Tennent and Baird, 1985). Coatings
- Page 354 and 355:
Table 8.2 Copper corrosion products
- Page 356 and 357:
Table 8.5 Galvanic series in seawat
- Page 358 and 359:
occur either as a result of the des
- Page 360 and 361:
colour of a decorative paint surfac
- Page 362 and 363:
traditional furniture materials. Po
- Page 364 and 365:
preferentially absorb energy at wav
- Page 366 and 367:
may not be directly proportional to
- Page 368 and 369:
Figure 8.4 Detail of a pine panel f
- Page 370 and 371:
Figure 8.6 Detail of the lower part
- Page 372 and 373:
(a) (b) to changes in the medium us
- Page 374 and 375:
which property changes become appar
- Page 376 and 377:
ond with the support. Uneven drying
- Page 378 and 379:
the structure of the lacquer film a
- Page 380 and 381:
8.10 Adhesives In practice, adhesiv
- Page 382 and 383:
~CH 2—CH—CH 2~ | O Alkoxy radic
- Page 384 and 385:
within the leather leads to a react
- Page 386 and 387:
crease at corners of upholstery, or
- Page 388 and 389:
eaves of buildings are a source fro
- Page 390 and 391:
inter-chain interactions are the ma
- Page 392 and 393:
(a) (b) Figure 8.13 Degradation of
- Page 394 and 395:
trims to the frame may render fibre
- Page 396 and 397:
Wessell (eds), Deterioration of Mat
- Page 398 and 399:
Shashoua, Y. (1996) A passive appro
- Page 400 and 401:
Part 4 Conservation
- Page 402 and 403:
9 Conservation preliminaries This c
- Page 404 and 405:
techniques and rigorous grounding i
- Page 406 and 407:
• The retention of as much origin
- Page 408 and 409:
(a) (b) (c) (d) (e) Conservation pr
- Page 410 and 411:
and preservation. He proposed a mod
- Page 412 and 413:
cally be undertaken. Before embarki
- Page 414 and 415:
cal, solutions include enclosing th
- Page 416 and 417:
various materials that make up the
- Page 418 and 419:
the nature, extent, severity and lo
- Page 420 and 421:
treatment needs. Reliable identific
- Page 422 and 423:
Figure 9.2 A good quality loupe (ri
- Page 424 and 425:
information should always be carefu
- Page 426 and 427:
ange immediately upon excitation. T
- Page 428 and 429:
when dyes are being used to achieve
- Page 430 and 431:
cause inaccurate readings. Wood tha
- Page 432 and 433:
structure of elements that have bee
- Page 434 and 435:
(a) ‘reading’ of the object. Fo
- Page 436 and 437:
design. This can be specially commi
- Page 438 and 439:
candle 1900 K Household electric li
- Page 440 and 441:
fessional quality shots in conserva
- Page 442 and 443:
• Availability of adaptors for cl
- Page 444 and 445:
Recording and reporting treatment O
- Page 446 and 447:
should also be provided in or close
- Page 448 and 449:
it is possible to share some facili
- Page 450 and 451:
Solvent storage Solvents should be
- Page 452 and 453:
(a) (b) and condition of incoming a
- Page 454 and 455:
Wherever possible, manual handling
- Page 456 and 457:
some specific details of that proce
- Page 458 and 459:
outes of entry to the body; risk ph
- Page 460 and 461:
(a) (b) Figure 9.11 Examples of app
- Page 462 and 463:
isks so that harm is unlikely? Only
- Page 464 and 465:
general maintenance of the workshop
- Page 466 and 467:
and other emergencies such as gas l
- Page 468 and 469:
Dunbar, M. (1989) Restoring, Tuning
- Page 470 and 471:
Stevens, R.E. (1980) Microscopical
- Page 472 and 473:
example, extensive treatment on one
- Page 474 and 475:
of the stock and a rule of thumb fo
- Page 476 and 477:
(a) (b) Chair leg False tenon 1/3 a
- Page 478 and 479:
(a) (b) (c) Figure 10.3 Testing the
- Page 480 and 481:
Hammer veneering with animal/hide g
- Page 482 and 483:
(a) (b) Principles of conserving an
- Page 484 and 485:
Table 10.2 Cramping/clamping device
- Page 486 and 487:
Table 10.3 Abrasives Principles of
- Page 488 and 489:
Table 10.3 Abrasives - continued ne
- Page 490 and 491:
measure the diagonals across the se
- Page 492 and 493:
(i) (ii) (a) Figure 10.11 Dismantli
- Page 494 and 495:
10.2.4 Reinforcing joints If a loos
- Page 496 and 497:
microballoons. They may be levelled
- Page 498 and 499:
Figure 10.15 Jig used to keep fills
- Page 500 and 501:
as a gap filler in cases where a fa
- Page 502 and 503:
should be critically evaluated befo
- Page 504 and 505:
(a) (b) Figure 10.21 Marking out do
- Page 506 and 507:
to the substrate or by using paper
- Page 508 and 509:
e progressively tightened over the
- Page 510 and 511:
Boucher (1995) described a techniqu
- Page 512 and 513:
(a) (b) (c) Figure 10.23 Repairing
- Page 514 and 515:
ducing the curves on boulle and mar
- Page 516 and 517:
similar fabric is stretched across
- Page 518 and 519:
ures involved in preparing a copy o
- Page 520 and 521:
and capture a high degree of detail
- Page 522 and 523:
materials, used extensively by scul
- Page 524 and 525:
making material. Traditional releas
- Page 526 and 527:
The compo can be rolled out into a
- Page 528 and 529:
Properties, Chemistry and Preservat
- Page 530 and 531:
ehaviour of twentieth and twenty-fi
- Page 532 and 533:
(a) (b) (c) cleaned area should be
- Page 534 and 535:
Cleaning tests may embrace a wide r
- Page 536 and 537:
11.2 Mechanical cleaning Mechanical
- Page 538 and 539:
When a scalpel is used to pick away
- Page 540 and 541:
adhere the unwanted varnish or dirt
- Page 542 and 543:
USA solvents (also known as) TLV (A
- Page 544 and 545:
alent to low aromatic (c.17-20% aro
- Page 546 and 547:
R' | R - C O The presence of the c
- Page 548 and 549:
suppliers. Careful selection of sol
- Page 550 and 551:
agency within the Department of Lab
- Page 552 and 553:
There are four stages in the dissol
- Page 554 and 555:
Figure 11.10 The positions of some
- Page 556 and 557:
Table 11.2 Teas’ solvent referenc
- Page 558 and 559:
ethanol acetone white spirit Figure
- Page 560 and 561:
as odourless mineral spirits or whi
- Page 562 and 563:
effective in breaking down coatings
- Page 564 and 565:
11.4.3 Acids Organic acids have the
- Page 566 and 567:
keep the contact time of both clean
- Page 568 and 569:
The two primary factors in choosing
- Page 570 and 571:
Detergents Commercially viable synt
- Page 572 and 573:
(a) (b) Figure 11.19 Orientation of
- Page 574 and 575:
carbon rinse is required (Burnstock
- Page 576 and 577:
Table 11.8 Properties of some deter
- Page 578 and 579:
constant (k) (at standard temperatu
- Page 580 and 581:
Conditional stability constant (log
- Page 582 and 583:
solution and act as a buffer, e.g.
- Page 584 and 585:
Figure 11.24 Old residues of a coll
- Page 586 and 587:
General purpose protease gel 100 ml
- Page 588 and 589:
Wax pastes have traditionally been
- Page 590 and 591:
on the application. It has been sug
- Page 592 and 593:
As with gels formulated from cellul
- Page 594 and 595:
Leonard, M., Whitten, J., Gamblin,
- Page 596 and 597:
Viscosity may be manipulated to mee
- Page 598 and 599:
12.2.2 Penetration of consolidant a
- Page 600 and 601:
Natural materials Wax has been used
- Page 602 and 603:
tive. Volatile ‘binding’ media
- Page 604 and 605:
that influences the choice of gelat
- Page 606 and 607:
malachite and azurite. Decorative s
- Page 608 and 609:
of flakes and cups but care should
- Page 610 and 611:
damage when levelling the fill and
- Page 612 and 613:
erty of the adhesive polymer rather
- Page 614 and 615:
dimensional forms such as curved co
- Page 616 and 617:
(a) (b) (c) (d) Principles of c
- Page 618 and 619:
chemical stability, flexibility and
- Page 620 and 621:
colours are ideal for under-saturat
- Page 622 and 623:
and those that must withstand use.
- Page 624 and 625:
efractive index for air is 1.003 (B
- Page 626 and 627:
use whenever possible and many cons
- Page 628 and 629:
tic, dammar and MS2A. Window glass
- Page 630 and 631:
although slower evaporating solvent
- Page 632 and 633:
ut insoluble in acetone and lower a
- Page 634 and 635:
Figure 12.13 Diagrammatic represent
- Page 636 and 637:
(a) (b) ‘touch-up’ guns may be
- Page 638 and 639:
appearance of paintings, Studies in
- Page 640 and 641:
American Chemical Society, Washingt
- Page 642 and 643:
often rely on self-levelling spray
- Page 644 and 645:
ecoming progressively harder and da
- Page 646 and 647:
Identifying a historical progressio
- Page 648 and 649:
normally seen, so that it can be mo
- Page 650 and 651:
Figure 13.4 Parafilm ® is a thin w
- Page 652 and 653:
of an overall finishing strategy. I
- Page 654 and 655:
e used cautiously and with suitable
- Page 656 and 657:
(a) (b) (c) Figure 13.6 The use of
- Page 658 and 659:
Figure 13.7 Proprietary shellac sti
- Page 660 and 661:
Thus conservation grade materials m
- Page 662 and 663:
ing the colour. This may be useful
- Page 664 and 665:
materials is their photochemical st
- Page 666 and 667:
(a) (b) Figure 13.11 Use of oils (a
- Page 668 and 669:
French polishing French polishing d
- Page 670 and 671:
during a polishing session. If the
- Page 672 and 673:
applying fresh polish before pullin
- Page 674 and 675:
the addition of a separate thixotro
- Page 676 and 677:
Roelop, W. (1994) Coloured mordants
- Page 678 and 679:
eflective shine, whilst oil gilding
- Page 680 and 681:
is used for laying gold and silver
- Page 682 and 683:
wood be dry, free of grime, oil and
- Page 684 and 685:
necessary to use a size coat as str
- Page 686 and 687:
however, makes it more difficult to
- Page 688 and 689:
flutes or other details in the carv
- Page 690 and 691:
14.2.12 Yellow ochre A coat of size
- Page 692 and 693:
applied only to highlight areas (Fi
- Page 694 and 695:
Figure 14.12 Manipulating the gold
- Page 696 and 697:
14.2.17 Double gilding Water gildin
- Page 698 and 699:
ground would be prepared uniformly
- Page 700 and 701:
areas, however deep, that have been
- Page 702 and 703:
15 Conserving other materials I Thi
- Page 704 and 705:
Consolidation Powdering or flaking
- Page 706 and 707:
synthetic materials, such as Paralo
- Page 708 and 709:
shell or horn itself may be dyed wi
- Page 710 and 711:
oration and brittleness. The cellul
- Page 712 and 713:
to reverse if necessary. Gelatin ma
- Page 714 and 715:
emoval. Dusting or wiping over the
- Page 716 and 717:
Figure 15.4 Reverse side of a music
- Page 718 and 719:
of electrolytes (e.g. after removin
- Page 720 and 721:
gases, vapours, liquids and ions. T
- Page 722 and 723:
when the object is handled. Researc
- Page 724 and 725:
and graphite. They were commonly us
- Page 726 and 727:
(a) (c) so if the treatment is to a
- Page 728 and 729:
into consideration the importance a
- Page 730 and 731:
Alkaline glycerol solution will rem
- Page 732 and 733:
Figure 15.11 A gold snuff box, c.18
- Page 734 and 735:
(1990) and CCI notes 9/7 (1993). Th
- Page 736 and 737:
into the workplace. The type of exp
- Page 738 and 739:
(a) (b) Figure 15.14 Use of pressur
- Page 740 and 741:
ensure that any water residues are
- Page 742 and 743:
The materials used in the decoratio
- Page 744 and 745:
solvent creeping behind or undernea
- Page 746 and 747:
Snow, C.E. and Weisser, T.D. (1984)
- Page 748 and 749:
Koob, S.P. (1986) The use of Paralo
- Page 750 and 751:
coatings that may have been applied
- Page 752 and 753:
acrylic paint may be used if it is
- Page 754 and 755:
16.2 Plastics 16.2.1 Introduction t
- Page 756 and 757:
PVAC, Paraloid B72, ethylene vinyl
- Page 758 and 759:
dyed by contract. Although this may
- Page 760 and 761:
Textiles used in upholstery conserv
- Page 762 and 763:
springs, iron tacks, or brass naili
- Page 764 and 765:
Reapplication of lined textiles Sta
- Page 766 and 767:
16.4 Leather, parchment and shagree
- Page 768 and 769:
minium triformate or aluminium alko
- Page 770 and 771:
(1991) reported that stronger bonds
- Page 772 and 773:
y enzymatic or lime action and whic
- Page 774 and 775:
The principles outlined above for s
- Page 776 and 777:
Figure 16.12 Front rail of a ninete
- Page 778 and 779:
may be appropriate for leather lini
- Page 780 and 781:
If the surface is unaffected by spo
- Page 782 and 783:
cation and characterization of the
- Page 784 and 785:
may not be representative of the ki
- Page 786 and 787:
Deoxycholate soap recipe 2 g deoxyc
- Page 788 and 789:
that which occurs during the remova
- Page 790 and 791:
(a) (b) (c) (d) 16.7.3 Cleaning [1]
- Page 792 and 793:
In the past, abrasives such as rott
- Page 794 and 795:
Wax has been used many times to inf
- Page 796 and 797:
permanently etch the lacquer, leavi
- Page 798 and 799:
etouching and coating of lacquer ob
- Page 800 and 801:
(a) (b) Figure 16.23 Japanese inro
- Page 802 and 803:
Extended exposure to polar solvents
- Page 804 and 805:
Retouching may involve reproducing
- Page 806 and 807:
ethical considerations. Objects tha
- Page 808 and 809:
tion will need to be reduced accord
- Page 810 and 811:
(a) (b) Figure 16.28 Filling with a
- Page 812 and 813:
to worked through the gold leaf slo
- Page 814 and 815:
Down, J.L., MacDonald, M.A., Willia
- Page 816 and 817:
Rococo Lacquers, Arbeitshefte des B
- Page 818 and 819:
Budden, S. (ed.) (1991) Gilding and
- Page 820 and 821:
Index Aalto, Alvar, 36, 38 Abalone
- Page 822 and 823:
Cabinetscraper, 449 Cabriole leg, 2
- Page 824 and 825:
Comparison, 385 Composites, 129 Com
- Page 826 and 827:
Electromotive force (EMF) series, 3
- Page 828 and 829:
deterioration, 333-5 gesso grounds,
- Page 830 and 831:
textiles, 351 wood, 290-1 for photo
- Page 832 and 833:
extenders, 145 identification, 187,
- Page 834 and 835:
plastics, 721 See also Coatings Rev
- Page 836 and 837:
Textiles, 107-12, 120 conservation,
- Page 838 and 839:
epair material selection, 437-9 woo
- Page 840 and 841:
(a) (c) Plate 1 Detail of leather u
- Page 842 and 843:
Plate 3 Side table (c.1775) designe
- Page 844 and 845:
(a) (b) Plate 6 A tall case japanne










![Til]tl](https://img.yumpu.com/45878240/1/190x245/tiltl.jpg?quality=85)




