Labelling Review row-Online
Labelling Review row-Online
Labelling Review row-Online
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Primary Antibodies<br />
Caspase-8<br />
Clone 11B6<br />
1 mL, 0.1 mL lyophilized NCL-CASP-8 F P (HIER)<br />
The caspases represent a family of cysteine proteases that play important<br />
regulatory roles within the cell. Caspase-8, also called FLICE, has an Nterminal<br />
domain with sequence homology to the death effector domain of<br />
FADD that allows association of caspase-8 with the TNF/Fas family of<br />
receptors. This association with the cell surface death receptors has shown<br />
caspase-8 to be a proximal regulator of apoptosis. Caspase-8 is activated by<br />
association with the Fas/FADD death-inducing signalling complex to release<br />
two active subunits, p18 and p10, into the cytosol, where they activate other<br />
caspases amplifying the apoptotic signal. Caspase-8 is reported to be<br />
expressed in pancreatic tumors, high grade non-Hodgkin's lymphomas and<br />
invasive breast carcinomas.<br />
Product Specific Information<br />
NCL-CASP-8 is raised to the p18 subunit found in caspases 8a, 8b and 8h.<br />
Caspase-9<br />
Clone 2C9B11<br />
1 mL, 0.1 mL lyophilized NCL-CASP-9 P (HIER) W<br />
Caspase-9 is a member of the caspase family of cysteine proteases that has<br />
been implicated in apoptosis and cytokine processing. Caspases have been<br />
shown to be activated during normal human keratinocyte differentiation and<br />
this activation is required for the normal loss of the nucleus. In addition, this<br />
apoptotic pathway may be activated in cardiac myocytes under conditions<br />
of ischemia. In the presence of ATP, apoptotic stimuli induce proteolytic<br />
processing and activation of pro-caspase 9 by cytochrome c and Apaf-1.<br />
Activated caspase-9 cleaves downstream caspases such as caspase-3, 6<br />
and 7 initiating the caspase cascade. Caspase-9 is essential for apoptosis<br />
during the normal development of the central nervous system. Mutations or<br />
deficiencies in caspase-9 result in resistance to apoptotic stimuli that mimic<br />
conditions in developing tumors.<br />
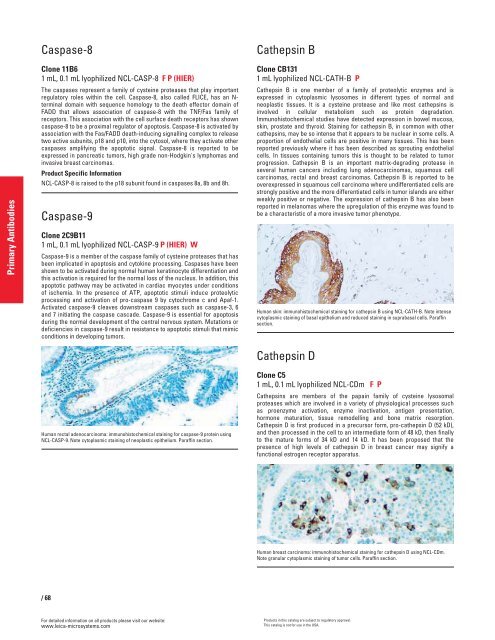
Human rectal adenocarcinoma: immunohistochemical staining for caspase-9 protein using<br />
NCL-CASP-9. Note cytoplasmic staining of neoplastic epithelium. Paraffin section.<br />
/68<br />
For detailed information on all products please visit our website:<br />
www.leica-microsystems.com<br />
Cathepsin B<br />
Clone CB131<br />
1 mL lyophilized NCL-CATH-B P<br />
Cathepsin B is one member of a family of proteolytic enzymes and is<br />
expressed in cytoplasmic lysosomes in different types of normal and<br />
neoplastic tissues. It is a cysteine protease and like most cathepsins is<br />
involved in cellular metabolism such as protein degradation.<br />
Immunohistochemical studies have detected expression in bowel mucosa,<br />
skin, prostate and thyroid. Staining for cathepsin B, in common with other<br />
cathepsins, may be so intense that it appears to be nuclear in some cells. A<br />
proportion of endothelial cells are positive in many tissues. This has been<br />
reported previously where it has been described as sprouting endothelial<br />
cells. In tissues containing tumors this is thought to be related to tumor<br />
progression. Cathepsin B is an important matrix-degrading protease in<br />
several human cancers including lung adenocarcinomas, squamous cell<br />
carcinomas, rectal and breast carcinomas. Cathepsin B is reported to be<br />
overexpressed in squamous cell carcinoma where undifferentiated cells are<br />
strongly positive and the more differentiated cells in tumor islands are either<br />
weakly positive or negative. The expression of cathepsin B has also been<br />
reported in melanomas where the upregulation of this enzyme was found to<br />
be a characteristic of a more invasive tumor phenotype.<br />
Human skin: immunohistochemical staining for cathepsin B using NCL-CATH-B. Note intense<br />
cytoplasmic staining of basal epithelium and reduced staining in suprabasal cells. Paraffin<br />
section.<br />
Cathepsin D<br />
Clone C5<br />
1 mL, 0.1 mL lyophilized NCL-CDm FP<br />
Cathepsins are members of the papain family of cysteine lysosomal<br />
proteases which are involved in a variety of physiological processes such<br />
as proenzyme activation, enzyme inactivation, antigen presentation,<br />
hormone maturation, tissue remodelling and bone matrix resorption.<br />
Cathepsin D is first produced in a precursor form, pro-cathepsin D (52 kD),<br />
and then processed in the cell to an intermediate form of 48 kD, then finally<br />
to the mature forms of 34 kD and 14 kD. It has been proposed that the<br />
presence of high levels of cathepsin D in breast cancer may signify a<br />
functional estrogen receptor apparatus.<br />
Human breast carcinoma: immunohistochemical staining for cathepsin D using NCL-CDm.<br />
Note granular cytoplasmic staining of tumor cells. Paraffin section.<br />
Products in this catalog are subject to regulatory approval.<br />
This catalog is not for use in the USA.