Chemická termodynamika II
Chemická termodynamika II
Chemická termodynamika II
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
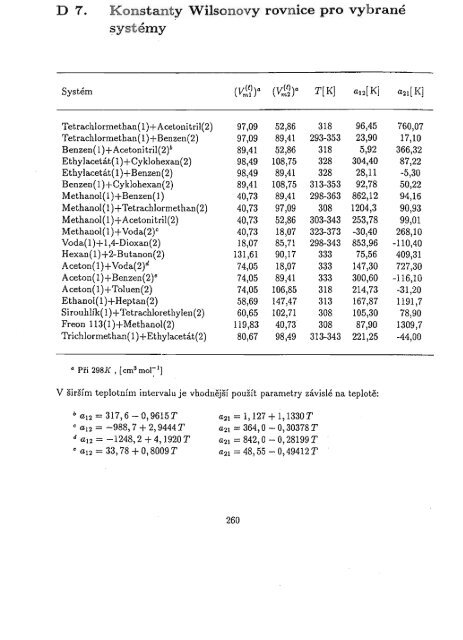
D 7.<br />
Konstanty Wilsonovy rovnice pro vyhrané<br />
~ystémy<br />
Systém (Vl?)a (Vl~)a T[K] adK] a21[KJ<br />
Tetrachlormethan(1)+Acetonitril(2) 97,09 52,86 318 96,45 760,07<br />
Tetrachlormethan(1)+ Benzen(2) 97,09 89,41 293-353 23,90 17,10<br />
Benzen(1)+Acetonitril(2)b 89,41 52,86 318 5,92 366,32<br />
Ethylacetát(l)+Cyklohexall(2) 98,49 108,75 328 304,40 87,22<br />
Ethylacetát(1)+Benzen(2) 98,49 89,41 328 28,11 -5,30<br />
Benzen(1)+Cyklohexan(2) 89,41 108,75 313-353 92,78 50,22<br />
Methanol(1)+Benzen(1) 40,73 89,41 298-363 862,12 94,16<br />
Methanol(I)+Tetrachlormethan(2) 40,73 97,09 308 1204,3 90,93<br />
Methanol(1)+Acetonitril(2) 40,73 52,86 303-343 253,78 99,01<br />
Methanol(l)+Voda(2)C 40,73 18,07 323-373 -30,40 268,10<br />
Voda(1)+1,4-Dioxan(2) 18,07 85,71 298-343 853,96 -110,40<br />
Hexan(l)+2-Butanon(2) 131,61 90,17 333 75,56 409,31<br />
Aceton(l)+Voda(2)d 74,05 18,07 333 147,30 727,30<br />
Aceton(1)+Benzen(2Y 74,05 89,41 333 300,60 -116,10<br />
Aceton(l)+Toluen(2) 74,05 106,85 318 214,73 -31,20<br />
Ethanol(l)+Heptan(2) 58,69 147,47 313 167,87 1191,7<br />
Sirouhlík(1)+Tetrachlorethylel1(2) 60,65 102,71 308 105,30 78,90<br />
Freon 113(1)+Methanol(2) 119,83 40,73 308 87,90 1309,7<br />
Trichlormethan(1)+Ethylacetát(2) 80,67 98,49 313-343 221,25 -44,00<br />
CI Při 298]( , [cOl 3 mol~ 1J<br />
V širším teplotním intervalu je vhodnější použít parametry závislé na teplotě:<br />
b a12 = 317,6 - 0,9615T<br />
c a12 = ......988,7 + 2,9444 T<br />
d a12 = -1248,2 + 4, 1920 T<br />
a21 = 1,127 + 1,1330 T<br />
a21 = 364, O- 0,30378 T<br />
a21 = 842, O- 0,28199 T<br />
e a12 = 33, 78 + O, 8009 T a21 = 48,55 - 0,49412 T<br />
260