30845 Suppl Giot.pdf - Giornale Italiano di Ortopedia e Traumatologia
30845 Suppl Giot.pdf - Giornale Italiano di Ortopedia e Traumatologia
30845 Suppl Giot.pdf - Giornale Italiano di Ortopedia e Traumatologia
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
As the implant provides an especially attractive surface for the<br />
attachment of bacteria, and hence promotes biofilm formation,<br />
it is possible that the biofilms form<br />
faster than the neutrophils can infiltrate<br />
and destroy the biofilm.<br />
With regard to the second question,<br />
a possible explanation is that this<br />
persistent biofilm infection elicits an<br />
ongoing neutrophil infiltration and<br />
activation. These neutrophils could<br />
then during the presumed phagocytosis<br />
– the main defence mechanism<br />
of neutrophils against inva<strong>di</strong>ng pathogens<br />
– release cytotoxic entities,<br />
which in turn could attack the host<br />
cells and tissues, a process also known<br />
as “frustrated phagocytosis” 18 19 . The<br />
“frustrated PMN”, but also dead and<br />
dying cells or the degraded tissue,<br />
would attract even more PMN, and<br />
also other cells of the immune system<br />
as reinforcement. In that situation two<br />
outcomes are possible: either the PMN<br />
together with the other cells of the<br />
immune response are now sufficiently<br />
activated to fight the biofilm resulting<br />
in a self-limitation of the local<br />
inflammatory response, or the host<br />
defence fails to clear the infection. In<br />
that case, the inflammatory reaction<br />
would progress, and tissue damage<br />
and loosening of the implant, would<br />
be the consequence. Of note, the<br />
proinflammatory environment might<br />
also attract monocytes as precursors<br />
of bone-degra<strong>di</strong>ng osteoclasts. There<br />
is evidence in the literature that proinflammatory<br />
me<strong>di</strong>ators might promote<br />
the generation of osteoclasts 20-25 . In<br />
that, the persistent biofilm infection<br />
could finally be linked to bone resorption,<br />
as it occurs in patients with<br />
implant-associated osteomyelitis.<br />
So far, the therapeutic options are<br />
rather limited. Attempts are made to<br />
select antibiotics or biocides that alone<br />
or in combination with other means,<br />
can detach, solubilise and eliminate<br />
established biofilms. Another major<br />
objective in research is the prevention<br />
of biofilm infection, e.g. by mo<strong>di</strong>fying<br />
the implanted materials or by impregnating<br />
them with antibiotics in order<br />
to inhibit attachment of bacteria and<br />
hence biofilm formation. Impregnated<br />
bone cement or silver-coated osteo-<br />
E. Meyle, G. M. Hänsch<br />
synthesis materials are now available; their efficacy in clinical<br />
settings is now being tested.<br />
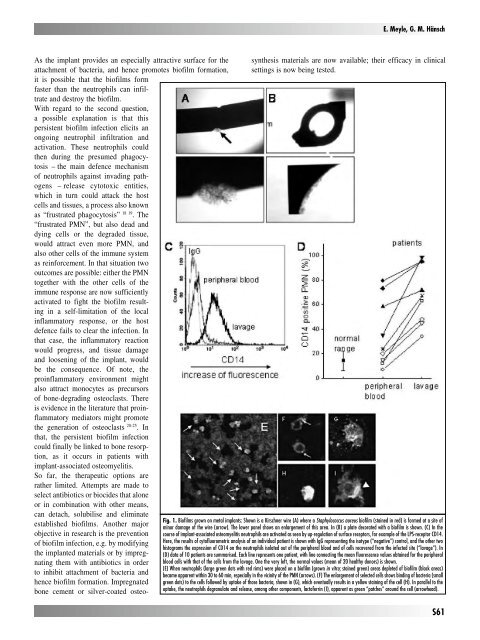
Fig. 1. Biofilms grown on metal implants: Shown is a Kirschner wire (A) where a Staphylococcus aureus biofilm (stained in red) is formed at a site of<br />
minor damage of the wire (arrow). The lower panel shows an enlargement of this area. In (B) a plate decorated with a biofilm is shown. (C) In the<br />
course of implant-associated osteomyelitis neutrophils are activated as seen by up-regulation of surface receptors, for example of the LPS-receptor CD14.<br />
Here, the results of cytofluorometric analysis of an in<strong>di</strong>vidual patient is shown with IgG representing the isotype (“negative”) control, and the other two<br />
histograms the expression of CD14 on the neutrophils isolated out of the peripheral blood and of cells recovered from the infected site (“lavage”). In<br />
(D) data of 10 patients are summarised. Each line represents one patient, with line connecting the mean fluorescence values obtained for the peripheral<br />
blood cells with that of the cells from the lavage. One the very left, the normal values (mean of 20 healthy donors) is shown.<br />
(E) When neutrophils (large green dots with red rims) were placed on a biofilm (grown in vitro; stained green) areas depleted of biofilm (black areas)<br />
became apparent within 30 to 60 min, especially in the vicinity of the PMN (arrows). (F) The enlargement of selected cells shows bin<strong>di</strong>ng of bacteria (small<br />
green dots) to the cells followed by uptake of those bacteria, shown in (G), which eventually results in a yellow staining of the cell (H). In parallel to the<br />
uptake, the neutrophils degranulate and release, among other components, lactoferrin (I), apparent as green “patches” around the cell (arrowhead).<br />
S61